When you think of spinach, you probably picture a leafy green superfood packed with iron, not a revolutionary tool in cardiac medicine. Yet, in one of the most fascinating intersections of nature and science, spinach leaves are now being used to grow artificial heart tissue — and the implications could be life-changing.
The Problem: Creating Blood Vessels in Lab-Grown Tissues
One of the most significant challenges in regenerative medicine and tissue engineering is developing functional blood vessels, especially when attempting to grow heart tissue in the lab. The human heart is an incredibly demanding organ, with every cell relying on a constant and efficient supply of oxygen and nutrients to function properly. This vital supply is delivered through a complex network of arteries, veins, and capillaries. Without such a network, even the most advanced synthetic tissues cannot survive for long, as cells begin to deteriorate and die within minutes in the absence of oxygen.
In natural tissues, blood vessels do much more than just transport nutrients. They play an essential role in maintaining cellular health, clearing away waste products, supporting immune system access, and ensuring overall tissue functionality. When these vessels are missing or poorly formed in lab-grown tissues, the constructs quickly become non-viable, especially in energy-intensive organs like the heart.
Researchers have explored various techniques to overcome this barrier. Some have attempted to 3D-print tiny vascular networks, while others have used microfluidic devices or seeded scaffolds with endothelial cells—the cells that naturally line human blood vessels. However, these methods often fall short. They may lack the ultra-fine branching patterns of natural capillaries, prove to be biologically unstable, or simply be too costly and complex to scale effectively.
This persistent problem has led scientists to look beyond traditional biomedical solutions—and surprisingly, toward the plant kingdom for inspiration. Among the most promising discoveries is the use of spinach leaves, whose natural vein structure offers a remarkably close match to the vascular systems in human tissue.
The Unexpected Solution: Spinach Leaves
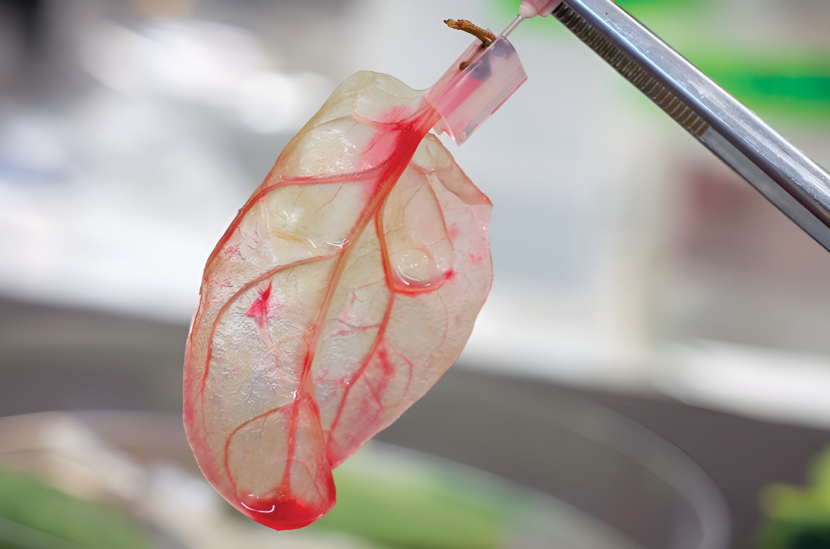
In a surprising twist of innovation, researchers found an unlikely ally in the fight to engineer functional heart tissue: the humble spinach leaf. At first glance, spinach may seem like nothing more than a leafy green vegetable, but its natural structure holds remarkable potential for medical science. The leaf’s vein network, responsible for transporting water and nutrients throughout the plant, closely resembles the branching pattern of human capillaries. This similarity caught the attention of scientists seeking new ways to create blood vessels in lab-grown tissues.
To harness this natural architecture, researchers developed a method called decellularization. This process involves removing all the plant cells from the spinach leaf, leaving behind a translucent scaffold composed primarily of cellulose—a biocompatible and non-toxic material. What remains is a delicate but durable framework that retains the intricate vascular pattern of the original leaf. This structure is ideal for mimicking the way blood flows through human tissue.
Once the leaf has been decellularized, scientists can introduce human heart cells onto the scaffold. These cells begin to attach, grow, and even beat in unison, simulating the natural activity of heart muscle. Fluids can be pumped through the leaf’s preserved vascular network, effectively replicating blood flow. This innovative use of plant material allows researchers to overcome one of the greatest obstacles in tissue engineering: replicating the fine, complex network of blood vessels necessary to keep tissues alive.
What makes spinach particularly appealing is not just its structural compatibility, but also its abundance, affordability, and sustainability. Using plant-based scaffolds could significantly reduce the cost and complexity of producing bioengineered tissues, paving the way for broader applications in regenerative medicine. What was once considered only a source of dietary fiber may soon play a key role in saving human lives.
How It Works
The process of turning a spinach leaf into a scaffold for heart tissue may sound like science fiction, but it’s based on straightforward, elegant steps rooted in bioengineering. It begins with decellularization, a technique where the plant cells are carefully removed from the spinach leaf using a gentle detergent solution. This process washes away the living material, leaving behind only the structural framework of the leaf—composed primarily of cellulose, a natural, plant-derived polymer that is both strong and biocompatible with human cells.
What remains after decellularization is a ghostly, translucent version of the original leaf. Though it no longer contains any plant cells, it preserves the intricate network of veins that once transported water and nutrients. This network is remarkably similar in structure and scale to the human microvasculature, making it ideal for supporting the flow of fluids like blood or nutrient-rich solutions.
Scientists then seed the cellulose scaffold with human heart cells, such as cardiomyocytes or endothelial cells. These cells adhere to the scaffold’s surface and begin to grow, guided by the natural contours and channels of the leaf’s vascular structure. Under the right conditions, these cells can even begin to contract rhythmically, mimicking the beating of real heart tissue.
To complete the process, a perfusion system is used to pump nutrient fluids through the leaf’s preserved vein network. This simulates blood flow, helping the cells receive oxygen and nutrients while removing waste—just as it would occur in a living organ. Over time, this setup allows for the development of functional tissue that can potentially be used for research, drug testing, or even as a foundation for future cardiac implants.
This innovative approach demonstrates how blending the efficiency of nature with the precision of biomedical science can create entirely new possibilities in regenerative medicine.
Why This Matters
The use of spinach leaves in tissue engineering isn’t just a clever scientific trick—it represents a major breakthrough in solving one of regenerative medicine’s most persistent challenges. By using the natural vascular structure of plants, scientists have found a way to overcome the difficulty of creating micro-scale blood vessels, which are essential for keeping lab-grown tissues alive. This development could dramatically accelerate the creation of viable artificial organs, especially for patients suffering from heart disease, where damaged tissue often needs to be replaced or repaired. What makes this approach even more promising is its practicality. Spinach is inexpensive, abundant, and renewable, making it a sustainable material for large-scale applications. Moreover, plant-derived scaffolds are naturally biocompatible, reducing the risk of rejection or inflammation when implanted in the human body. In a field often limited by high costs, complexity, and resource shortages, this simple yet ingenious technique could pave the way for more accessible and affordable medical treatments—potentially saving countless lives in the process.
The Future of Bioengineering
The successful use of spinach leaves to support heart tissue growth is just the beginning of a broader movement toward plant-based bioengineering. Scientists are now exploring the vascular structures of various plants—such as parsley, bamboo, and even peanut roots—to mimic different types of human tissues and organs. The idea is simple but powerful: nature has already spent millions of years perfecting complex networks for fluid transport, and these systems can be repurposed to serve human biology. As this research progresses, we could one day see entire organs grown using plant scaffolds, dramatically reducing the need for donor organs and transforming the future of transplantation. These bioengineered tissues might also be used to test new drugs more accurately, personalize treatments, or repair injuries in ways that were once considered impossible. Ultimately, the fusion of plant structures and human cells represents a shift toward greener, more sustainable, and more accessible medical technologies—offering hope not only for patients with chronic disease but also for the entire healthcare system as it looks toward a regenerative future.
Final Thoughts
What began as a curious intersection between botany and biomedical science has now blossomed into one of the most promising frontiers in regenerative medicine. The use of spinach leaves to mimic human vasculature is a brilliant reminder that sometimes the answers to our most complex problems can be found in nature. This groundbreaking research not only offers a potential lifeline for patients awaiting heart repair or organ transplants, but it also points toward a more sustainable and innovative future in medical science. As we continue to explore the natural world for inspiration, it’s exciting to imagine what other plant-based solutions might help heal the human body. From humble leaves to lifesaving breakthroughs, the future of bioengineering is rooted in both creativity and the extraordinary designs already present in the world around us.